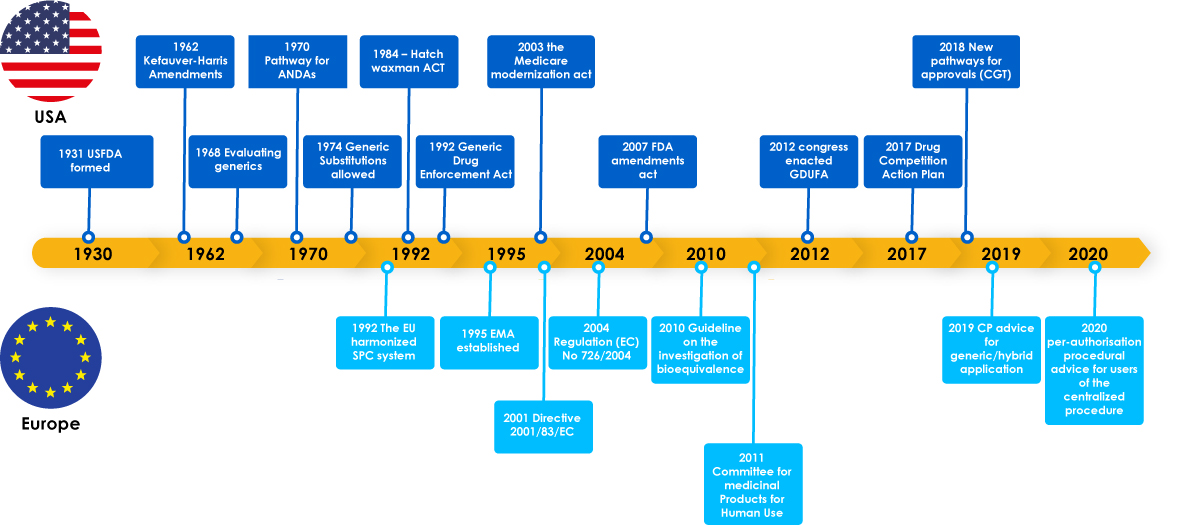
Generic medicines had major milestones including, Regulatory laws to support generic development, which encouraged manufacturers to explore the generics market. These legislations also provided the physicians a confidence to prescribe a substitute for a brand-name drug and it eventually benefitted patients, as generic manufacturers met critical needs of patients across the globe.
Evolution of Generics
FAQs on Generics
Q1: What are generic drugs?
Ans: Generic drugs are pharmaceutical drugs, which are identical copies of the originator drug product and have the same API, dosage, intended use, side effects, route of administration, risks, safety and strength as the innovator drug, but it may differ in some characteristics such as, the manufacturing process, formulation, excipients, colour, taste and packaging. In other words, a generic drug is therapeutically equivalent to a branded drug and therefore, can be substituted with its brand-name counterpart.
Q2: Why do generic drugs cost less than an innovator drug?
Ans: Generic drugs are more cost-effective when compared to the innovator drug, because generic drugs do not have to repeat the costly and lengthy pre-clinical and clinical studies, that are required for the innovator medicines to demonstrate safety and effectiveness and instead, the generic medicines need to prove only the pharmaceutical equivalency for their product.
Q3: Are generic drugs as good as innovator drug?
Ans: Yes, generic drugs have the same quality, efficacy and are safe as brand-name medicines. The lower price of these drugs doesn’t indicate that they are of inferior quality, because generic medicines just like brand-name drugs, have to go through a rigorous drug approval process to market their drug product and the pharmaceutical companies are required to submit the generic drug application, showing that, their drug product has the same clinical benefit as the brand-name medicines and are suitable to be used as a substitute for their respective brand-name drug.
Q4: Why do generic drugs look different than the brand-name drug product?
Ans: Generic drug products must be bioequivalent to branded drugs and have the same API as Innovator drugs. However, there can be minute differences in the generic drug products w.r.t shape, labeling (minor differences), packaging and inactive ingredients like, color, flavors and preservatives; but the effectiveness of the drug must remain the same.
Q5: What’s involved in reviewing and approving generic drug applications in the US?
Ans: Any pharmaceutical company that has to market its generic drug product in the US should comply with all the US FDA’s Regulatory requirements for generic drug filing and must show that:
- The generic drug is “Pharmaceutically/Therapeutically equivalent” to the brand
- The manufacturer is capable of making the drug correctly and consistently
- The “active ingredient” is same as that of the brand
- The right amount of the active ingredient gets to the place in the body, where it has an effect
- The "inactive" ingredients of the drug are safe
- The drug does not break down over time
- The container in which the drug will be shipped and sold is appropriate
- The label is same as the brand-name drug’s label
- Relevant patents or legal exclusivities are expired
Q6: Does every brand-name drug have a generic drug?
Ans: No. Every brand-name drug doesn’t have the generic version. New drugs are manufactured under patent protection for up to twenty (20) years. This means that no other drug can be manufactured and marketed during this period, until the expiration of the patent. However, some drugs may never have generic versions for their product, due to difficulty in manufacturing or in case the generic drug product may deem to be unprofitable.